Company
Greetings

KMG Co., Ltd. is a company specializing in medical devices.
KMG Co., Ltd. started out as Ku Myoung Electronics, a company manufacturing and developing medical devices, in 1997 and has ceaselessly endeavored to make everyone’s life beautiful and healthy.
NO PAIN, Korea’s first pain-free anesthesia machine
NO PAIN is a pain-free anesthesia machine that was developed and produced for the first time in Korea. This product was developed while studying comfortable and safe dental treatment methods for children who fear dental treatment. This product has become so famous now that its name has turned into a proper noun.
In addition, KMG is expanding its businesses to offer happy healthcare lives for men and women of all ages, such as ICT and TENS equipment for the treatment of muscle pain and neural pain, life-friendly medical devices to enhance the health and welfare of the elderly, and medical and beauty equipment for healthy and beautiful lives.
Differentiated technologies and genuine products
KMG will strive to make everyone’s life beautiful and healthy with differentiated technologies and genuine products.
-
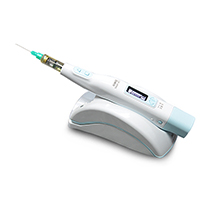
DENTAL & MEDICAL
We develop and produce injectors specialized in injecting anesthetics and drugs and manufacture and sell a variety of product groups that can effectively help dental treatment.
We develop, produce, and sell various electrical therapeutic devices to treat and alleviate pain, such as orthopedic, physical therapy, and oriental therapies.
-
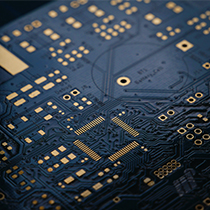
ELECTRONIC
SMD lines are operated to enable mass production of PCB.
We can design PCB and produce ZIG depending on the request of customers.
-

BEAUTY
Based on the technologies accumulated by developing medical devices, KMG develops, produces, and sells products effective for beauty care with the goal of leading the medical beauty market.
Organizational Status
-
CEO
-
Auditor
-
Affiliated Research CenterR&D Team
Medical Device SW Development
Medical Device Control Unit Development
R&D TeamMedical Device SW Development
Medical Device Control Unit Development
Production HeadquartersProduction TeamProduction Management
Inspection Management
Production Planning
QC Process Management
Material TeamSupplier Management
Purchase Management
Raw and Side Material Management
Finished Product Shipment
Production TeamCustomer Service
Non-conformity Management
Management Support HeadquartersGeneral Affairs and AccountingPersonnel
General Affairs
Finance/ Accounting
Logistics Management
PlanningMarketing Strategy Planning
New Business Strategy Planning
Financial Strategy Planning
Production Strategy Planning
Personnel Strategy Planning
Quality Control TeamProduct Certification Management
Facility Management
Education and Training Management
Internal Quality Audit
Sales HeadquartersSales TeamPublic Relations
Branch Management
Marketing Area Analysis
New Branch Opening
Overseas Export
Production Process
-
01
OEM·ODM
Customer’s production request/order -
02
Maintenance/ Management of production system
Maintenance of GMP, ISO 13485 certifications -
03
Operation of production management system
Optimal quality management -
04
Packaging and inspection
Performance/Safety inspection, use of packaging materials -
05
Delivery
Customer satisfaction -
06
Follow-up management
Customer management, customer service
SMT Process Flow
-
 01
01Print process
-
 02
02Chip mount process
-
 03
03Odd shape mount process
-
 04
04Re-flow process
-
 05
05Vision inspection
Quality feedback -
 06
06QC visual inspection
Repair if determined as defect -
 07
07Shipment